ADACCESS
A 51-week, double-blind, Phase 3 confirmatory study of patients with moderate-to-severe plaque psoriasis that assessed the impact of multiple switches between biosimilar adalimumab-adaz and reference adalimumab1

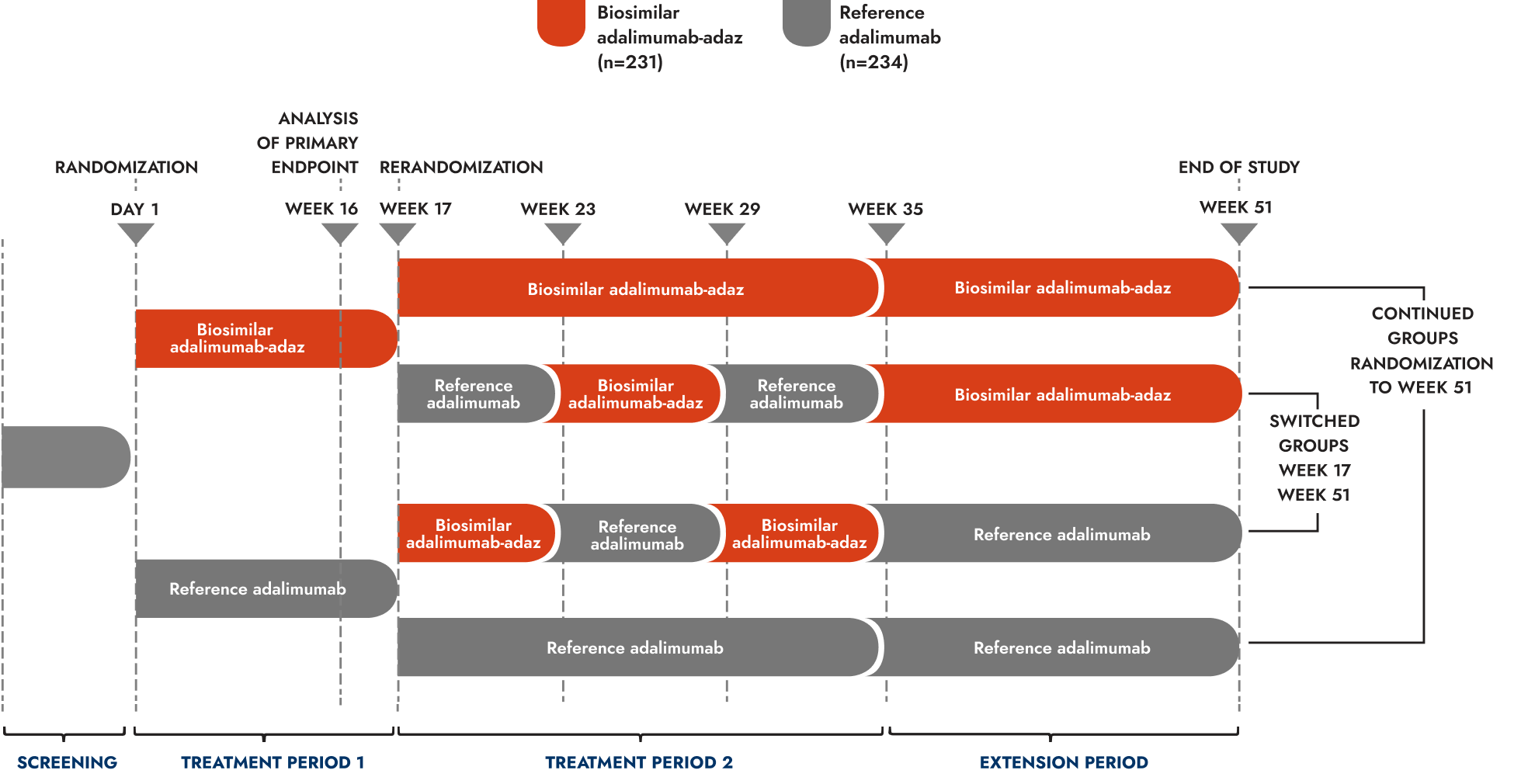
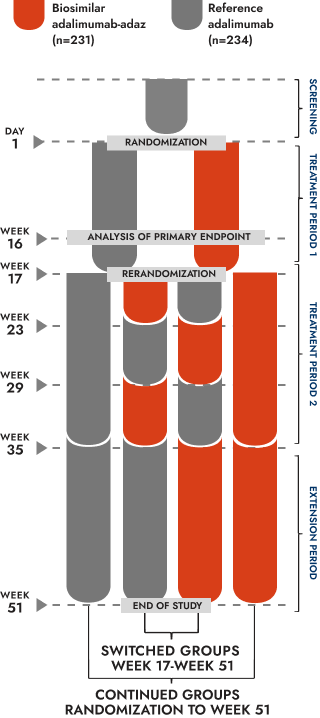
Treatment Period 1 (randomization to Week 17):
465 patients with active, clinically stable, moderate-to-severe plaque psoriasis randomized 1:1 to biosimilar adalimumab-adaz (n=231) or reference adalimumab (n=234). Patients received 80 mg of biosimilar adalimumab-adaz or reference adalimumab subcutaneously at Week 0, then 40 mg biweekly from Week 1 until Week 15
Treatment Period 2 (Weeks 17-35):
Patients achieving at least a PASI 50 response at Week 16 were rerandomized to continue their originally assigned treatment until Week 35 or receive either biosimilar adalimumab-adaz or reference adalimumab during 3 alternating 6–week periods
Extension Period (Weeks 35-51):
All patients received the treatment originally assigned at randomization
Primary Endpoint
- Proportion of patients who achieved PASI 75 at Week 16
- Equivalence was confirmed if the 95% confidence interval for the difference in PASI 75 response rates between treatments at Week 16 was contained within a margin of ±18%
PASI=Psoriasis Area and Severity Index.

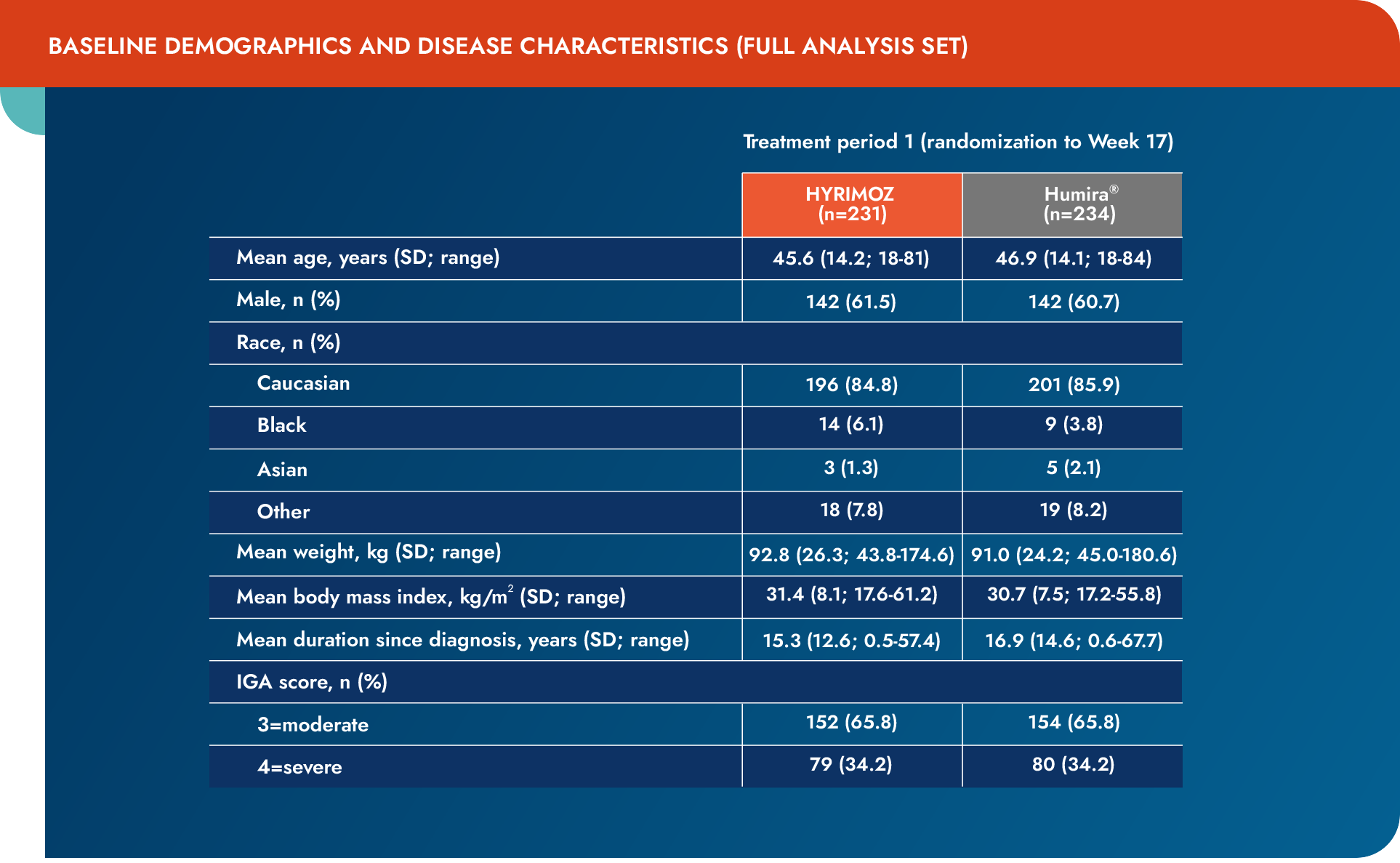
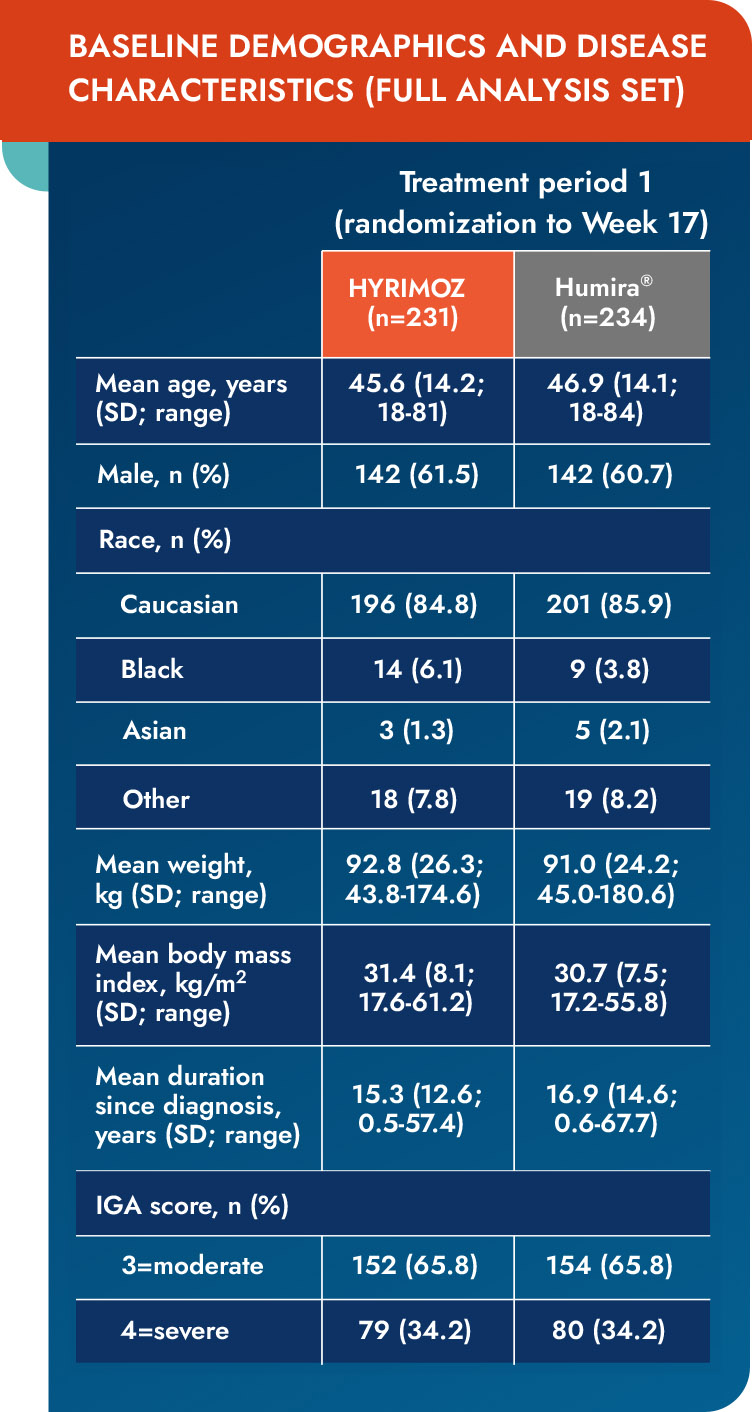
IGA=Investigator’s Global Assessment; SD=standard deviation.
Efficacy
Equivalent efficacy was shown between biosimilar adalimumab-adaz and reference adalimumab, with no impact from switching at 16 weeks1

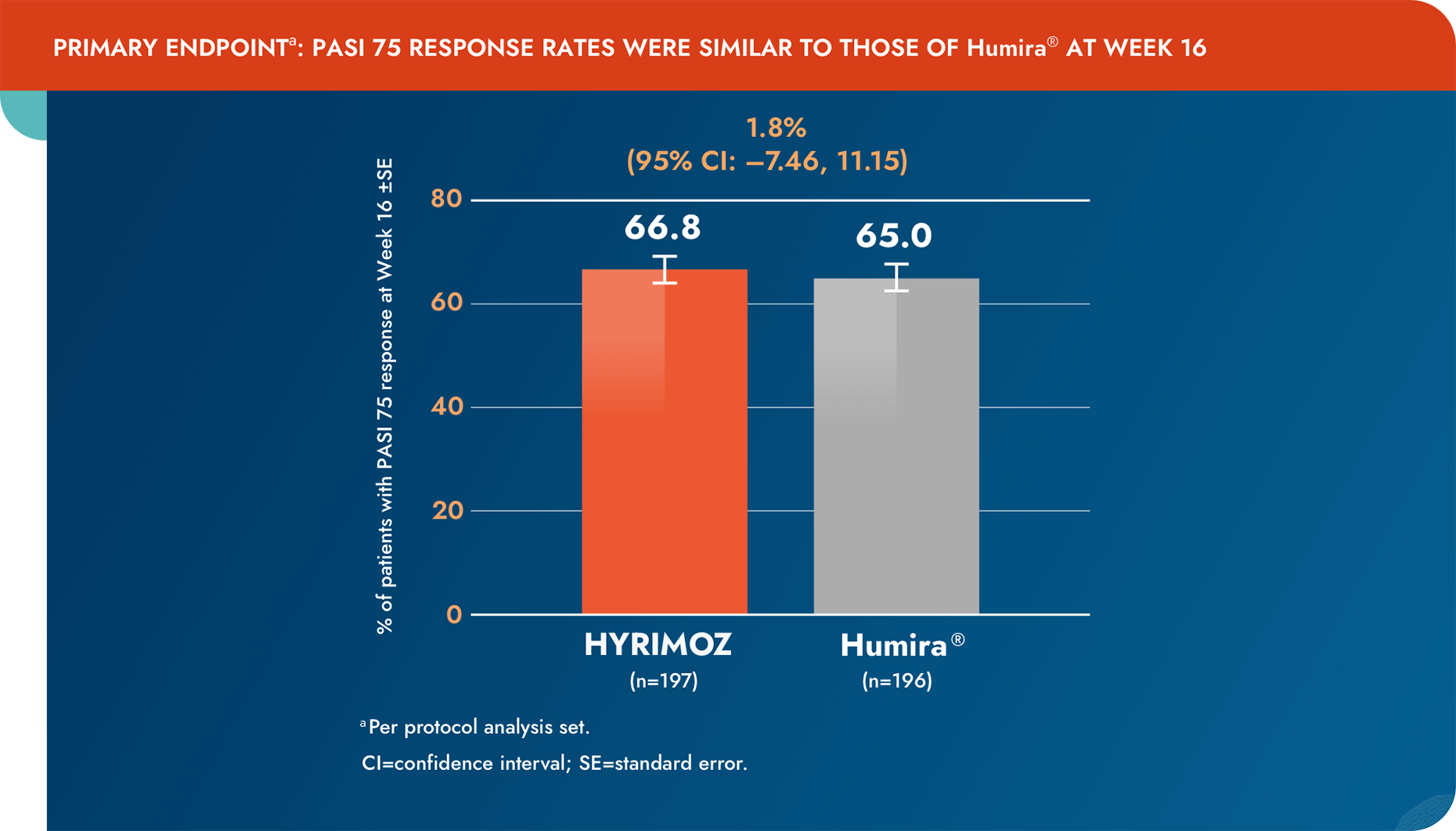
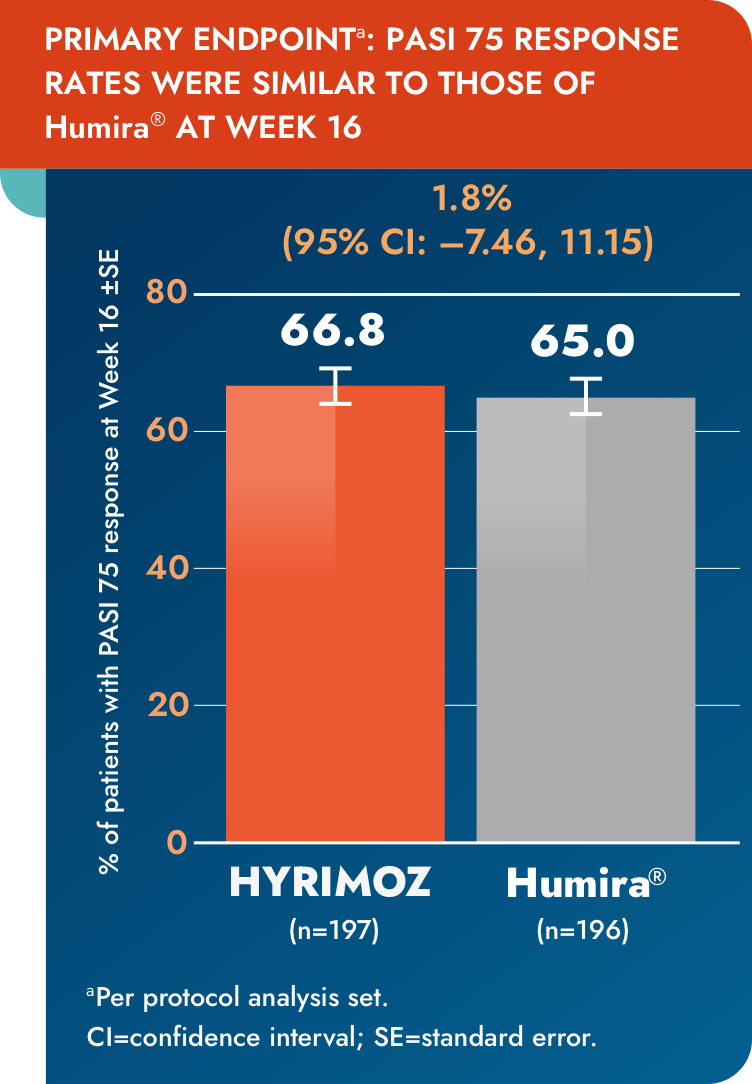
aPer protocol analysis set.
CI=confidence interval; PASI=Psoriasis Area and Severity Index; SE=standard error.
Safety
Switching up to 4 times between reference adalimumab and biosimilar adalimumab-adaz had no impact on the incidence of AEs, SAEs, or injection-site reactions1

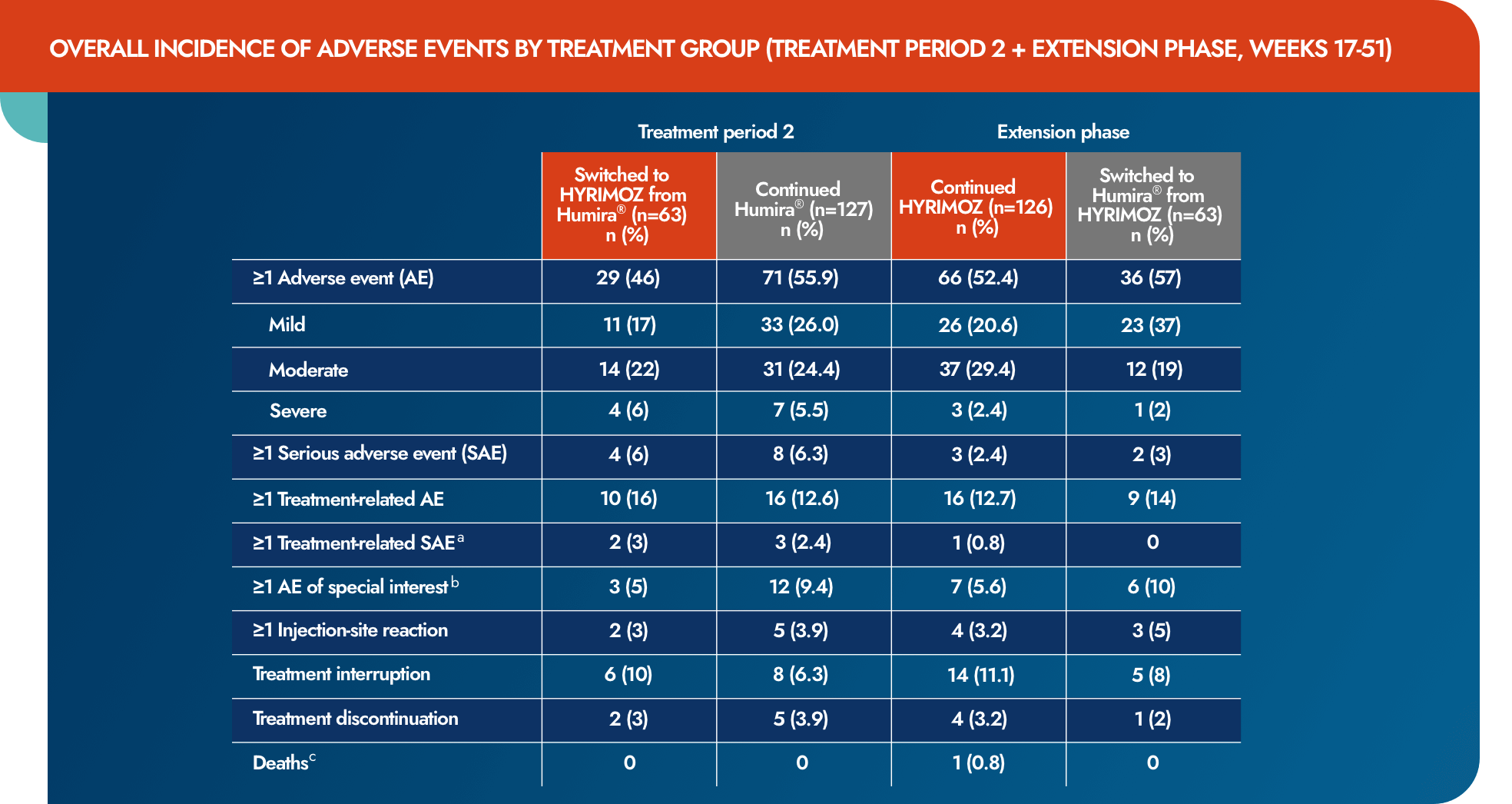
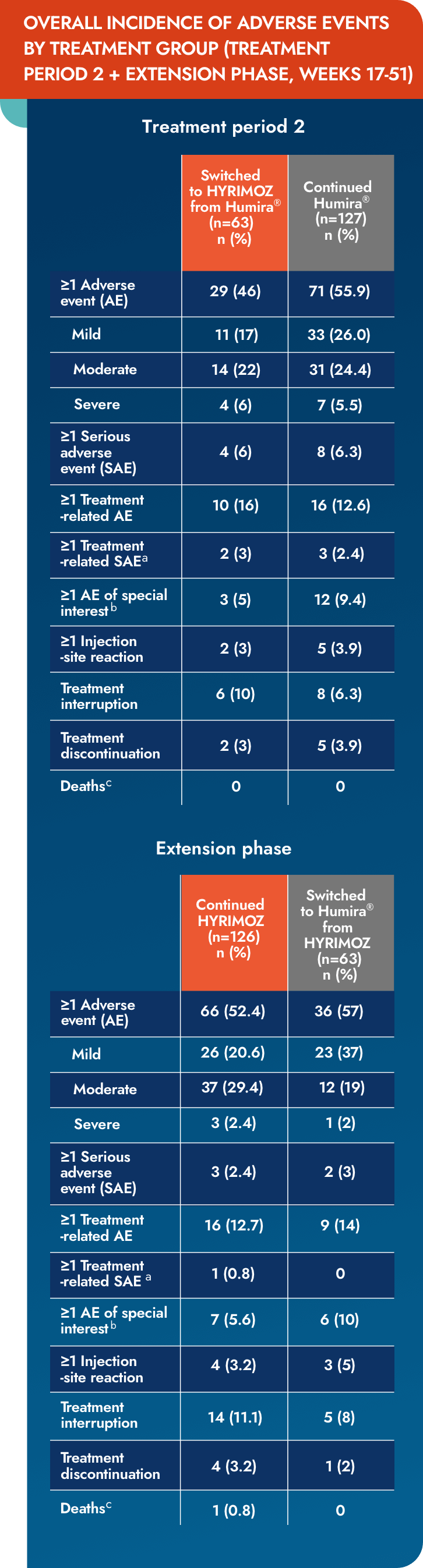
- No relevant differences in reporting of AEs and SAEs between treatment groups
- Rates of reported AEs were similar for most system organ classes and preferred terms
- Differences of >10% were seen in gastrointestinal disorders, which were reported by 3.2% of patients in the reference adalimumab to biosimilar adalimumab-adaz group compared with 14.2% of patients in the continued reference adalimumab group
aTen patients had an SAE that was considered treatment related, comprising severe toxic skin eruption, moderate pneumonia, severe cellulitis (reference adalimumab); severe staphylococcal infection and severe hypersensitivity (both in 1 patient; biosimilar adalimumab-adaz), severe pneumonia and moderate pyrexia (TP2 + EP, reference adalimumab to biosimilar adalimumab-adaz group), severe pulmonary tuberculosis, severe necrotizing pneumonia and moderate pneumonia (TP2 + EP, continued reference adalimumab group) and moderate pyelonephritis (TP2 + EP, continued biosimilar adalimumab-adaz group).
bEncompasses all the warnings and precautions given in the label for Humira® (adalimumab), including infections, malignancy, allergic or anaphylactic reactions, immune system disorders or autoimmune events, neurological events, hematological events, congestive heart failure, and interstitial lung disease.
cReported death in a patient with a history of depression, death due to suicide, not considered to be related to study treatment.
AE=adverse event; EP=extension period; SAE=serious adverse event; TP=treatment period.
Immunogenicity
Similar frequency of development of ADAs across switched and continued groups1

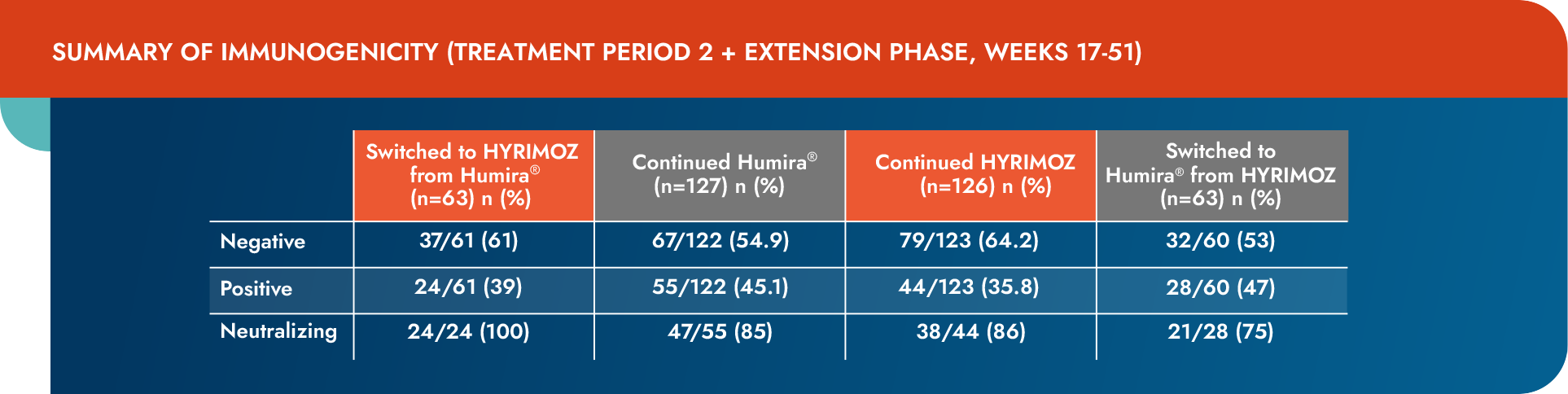
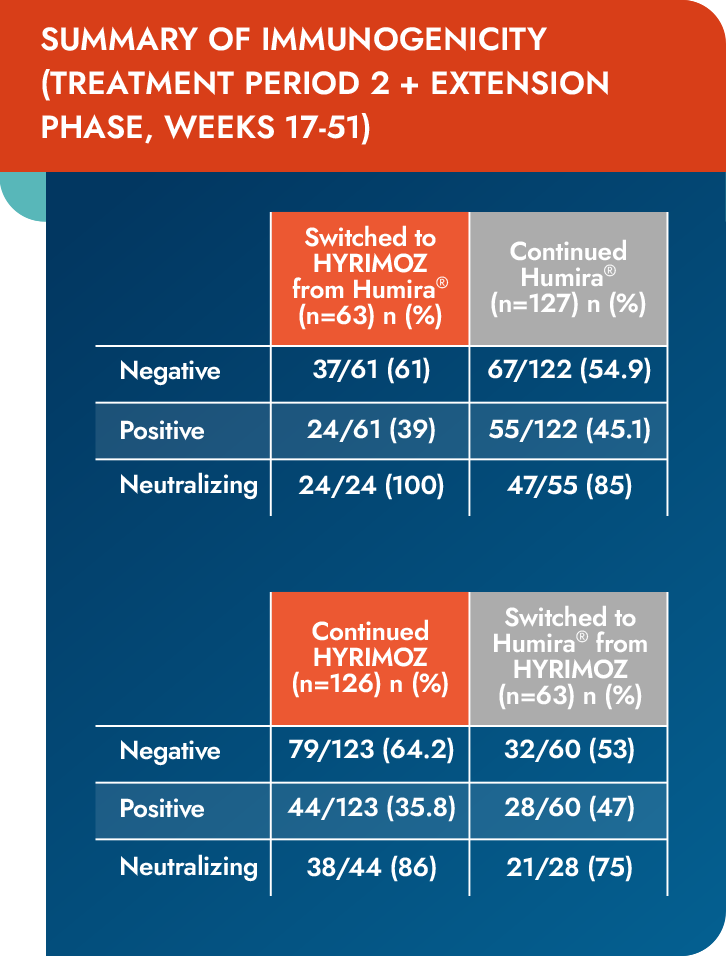
Values are the antidrug antibody detection rate, n/N (%).
- During treatment period 1, the proportion of patients reporting ≥1 positive ADA response up to Week 17 was 36.8% and 34.1% in the biosimilar adalimumab-adaz and reference adalimumab groups, respectively
ADA=antidrug antibody.

ADMYRA
The Sensoready® pen

Co-pay as low as $0
INDICATIONS: HYRIMOZ® (adalimumab-adaz) injection for subcutaneous use, a prescription medication, is a tumor necrosis factor (TNF)-blocker indicated for:
- Rheumatoid Arthritis (RA): Alone or in combination with methotrexate or other non-biologic disease-modifying anti-rheumatic drugs (DMARDs), for reducing signs and symptoms, inducing major clinical response, inhibiting the progression of structural damage, and improving physical function in adult patients with moderately to severely active RA
- Juvenile Idiopathic Arthritis (JIA): Alone or in combination with methotrexate for reducing signs and symptoms of moderately to severely active polyarticular JIA in patients 2 years of age and older
- Psoriatic Arthritis (PsA): Alone or in combination with non-biologic DMARDs, for reducing signs and symptoms, inhibiting the progression of structural damage, and improving physical function in adult patients with active PsA
- Ankylosing Spondylitis (AS): Reducing signs and symptoms in adult patients with active AS
- Crohn’s Disease (CD): Treatment of moderately to severely active CD in adults and pediatric patients 6 years of age and older
- Ulcerative Colitis (UC): Treatment of moderately to severely active UC in adult patients
Limitations of use: The effectiveness of adalimumab products has not been established in patients who have lost response to or were intolerant to TNF blockers. - Plaque Psoriasis (Ps): Treatment of adult patients with moderate to severe chronic Ps who are candidates for systemic therapy or phototherapy, and when other systemic therapies are medically less appropriate. HYRIMOZ should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician
- Hidradenitis Suppurativa (HS): Treatment of moderate to severe HS in adult patients
- Uveitis: Treatment of non-infectious intermediate, posterior, and panuveitis in adult patients
IMPORTANT SAFETY INFORMATION:
WARNING: SERIOUS INFECTIONS and MALIGNANCY
SERIOUS INFECTIONS
Patients treated with adalimumab products, including HYRIMOZ, are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue HYRIMOZ if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before HYRIMOZ use and during therapy. Initiate treatment for latent TB prior to HYRIMOZ use
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness
- Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria
Carefully consider the risks and benefits of treatment with HYRIMOZ prior to initiating therapy in patients with chronic or recurrent infection.
Monitor patients closely for the development of signs and symptoms of infection during and after treatment with HYRIMOZ, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers including adalimumab products. Post-marketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF-blockers including adalimumab products. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. Almost all these patients had received treatment with azathioprine or 6-mercaptopurine (6–MP) concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants.
WARNINGS AND PRECAUTIONS
Serious Infections
- Do not start HYRIMOZ during an active infection, including localized infections
- If an infection develops, monitor carefully, and stop HYRIMOZ if infection becomes serious. Drug interactions with biologic products: A higher rate of serious infections has been observed in RA patients treated with rituximab who received subsequent treatment with a TNF blocker. An increased risk of serious infections has been seen with the combination of TNF blockers with anakinra or abatacept, with no demonstrated added benefit in patients with RA. Concomitant administration of HYRIMOZ with other biologic DMARDs (eg, anakinra or abatacept) or other TNF blockers is not recommended based on the possible increased risk for infections and other potential pharmacological interactions
- Patients 65 years of age and older, patients with co-morbid conditions and/or patients taking concomitant immunosuppressants may be at greater risk of infection
- Invasive fungal infections: For patients who develop a systemic illness on HYRIMOZ, consider empiric antifungal therapy for those who reside or travel to regions where mycoses are endemic
Malignancies
- In clinical trials, incidence of malignancies was greater in adalimumab-treated patients than in controls
- Consider the risks and benefits of TNF blocker-treatment, including HYRIMOZ, prior to initiating therapy in patients with known malignancy
- Non-melanoma skin cancer (NMSC) was reported during clinical trials for adalimumab-treated patients. Examine all patients, particularly those with a history of prolonged immunosuppressant or PUVA therapy for the presence of NMSC prior to and during treatment with HYRIMOZ
- In the adalimumab clinical trials there was an approximate 3-fold higher rate of lymphoma than expected in the general US population. Patients with chronic inflammatory diseases, particularly those with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk than the general population for the development of lymphoma, even in the absence of TNF blockers
- Post-marketing cases of acute and chronic leukemia have been reported in association with TNF-blocker use. Approximately half of the post-marketing cases of malignancies in children, adolescents, and young adults receiving TNF blockers were lymphomas; other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents
Hypersensitivity Reactions
- Anaphylaxis or serious allergic reactions have been reported following administration of adalimumab products. If an anaphylactic or other serious hypersensitivity reaction occurs, immediately discontinue administration of HYRIMOZ and institute appropriate therapy
Hepatitis B Virus Reactivation
- Use of TNF blockers, including HYRIMOZ, may increase the risk of reactivation of hepatitis B virus (HBV) in patients who are chronic carriers. Some cases have been fatal
- Evaluate patients at risk for HBV infection for prior evidence of HBV infection before initiating TNF blocker therapy
- Exercise caution in patients identified as carriers of HBV and closely monitor during and after HYRIMOZ treatment
- In patients who develop HBV reactivation, stop HYRIMOZ and initiate effective anti-viral therapy. Exercise caution when resuming HYRIMOZ after HBV treatment
Neurologic Reactions
- Use of TNF-blocking agents, including adalimumab products, has been associated with rare cases of new onset or exacerbation of central nervous system and peripheral demyelinating disease, including multiple sclerosis (MS), optic neuritis, and Guillain-Barré syndrome
- Exercise caution when considering HYRIMOZ for patients with these disorders; discontinuation of HYRIMOZ should be considered if any of these disorders develop
- There is a known association between intermediate uveitis and central demyelinating disorders
Hematological Reactions
- Rare reports of pancytopenia, including aplastic anemia, have been reported with TNF-blocking agents. Medically significant cytopenia has been infrequently reported with adalimumab products
- Consider stopping HYRIMOZ if significant hematologic abnormalities occur
Heart Failure
- Worsening and new onset congestive heart failure (CHF) has been reported with TNF blockers. Cases of worsening CHF have also been observed with adalimumab products; exercise caution and monitor carefully
Autoimmunity
- Treatment with adalimumab products may result in the formation of autoantibodies and, rarely, in the development of a lupus-like syndrome. Discontinue treatment if symptoms of a lupus-like syndrome develop
Immunizations
- Patients on HYRIMOZ should not receive live vaccines
- Pediatric patients, if possible, should be brought up to date with all immunizations prior to initiating HYRIMOZ therapy
- Adalimumab is actively transferred across the placenta during the third trimester of pregnancy and may affect immune response in the in utero exposed infant. The safety of administering live or live-attenuated vaccines in infants exposed to adalimumab products in utero is unknown. Risks and benefits should be considered prior to vaccinating (live or live-attenuated) exposed infants
ADVERSE REACTIONS
The most common adverse reactions (incidence >10%): infections (eg, upper respiratory, sinusitis), injection site reactions, headache, and rash.
Please see full Prescribing Information for HYRIMOZ, including Boxed Warning and Medication Guide.
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc. at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.


